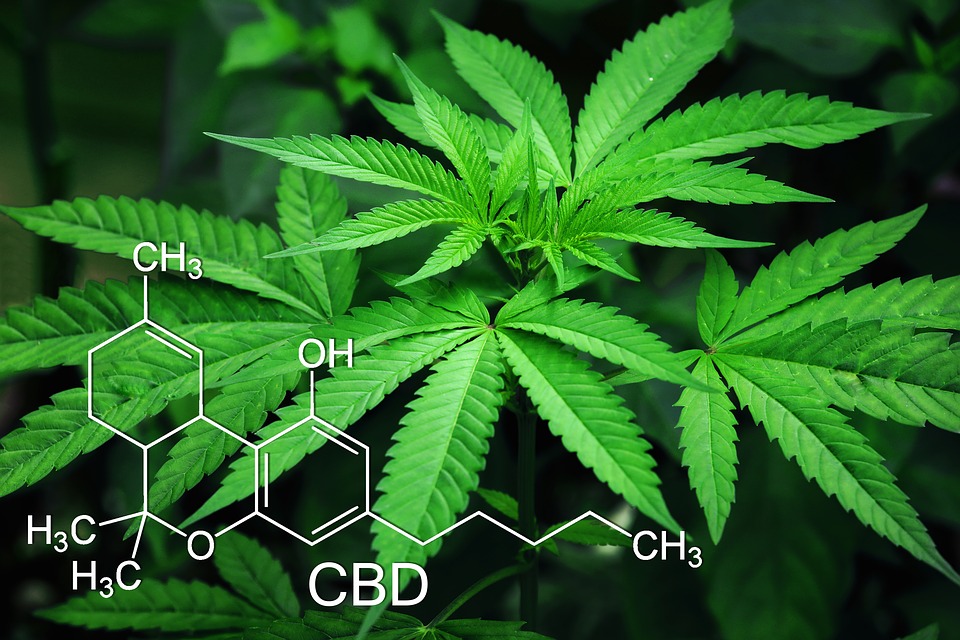
Epidiolex is now the only legal CBD oil recognized under federal law. CBD is the non-psychoactive cannabinoid derived from the hemp plant that many people use for its supposed therapeutic properties to treat several ailments and chronic illnesses. According to reports the 2018 U.S. Farm Bill is set to remove hemp from the Drug Enforcement Administration list of controlled substances. New sources have revealed that Epidiolex was approved this summer by the FDA and the U.S. Drug Enforcement Administration announced that Epidiolex has been classified as a Schedule V drug, legal for sale in the United States.
As a Schedule V drug, it will be classified as one of the least dangerous, least harmful and least addictive types of drugs on the market. A rescheduling order was also submitted for drugs that contain CBD oil and set to officially publish in the Federal Register on September 28, 2018.,
Excerpt from Federal Register:
Specifically, this order places FDA-approved drugs that contain CBD derived from cannabis and no more than 0.1 percent tetrahydrocannabinols in schedule V. This action is required to satisfy the responsibility of the Acting Administrator under the CSA to place a drug in the schedule he deems most appropriate to carry out United States obligations under the Single Convention on Narcotic Drugs, 1961. Also consistent therewith, DEA is adding such drugs to the list of substances that may only be imported or exported pursuant to a permit.
